Imagion Biosystems Limited (ASX: IBX), a company dedicated to improving healthcare through the earlier detection of cancer, is pleased to announce its plans to conduct its first-in-human study in Australia. The study will be the first time the MagSense® diagnostic imaging technology will be used in a clinical setting. Additionally, the Company intends to expand the study to investigate the utility of its nanoparticles as a Magnetic Resonance Imaging (MRI) contrast agent.
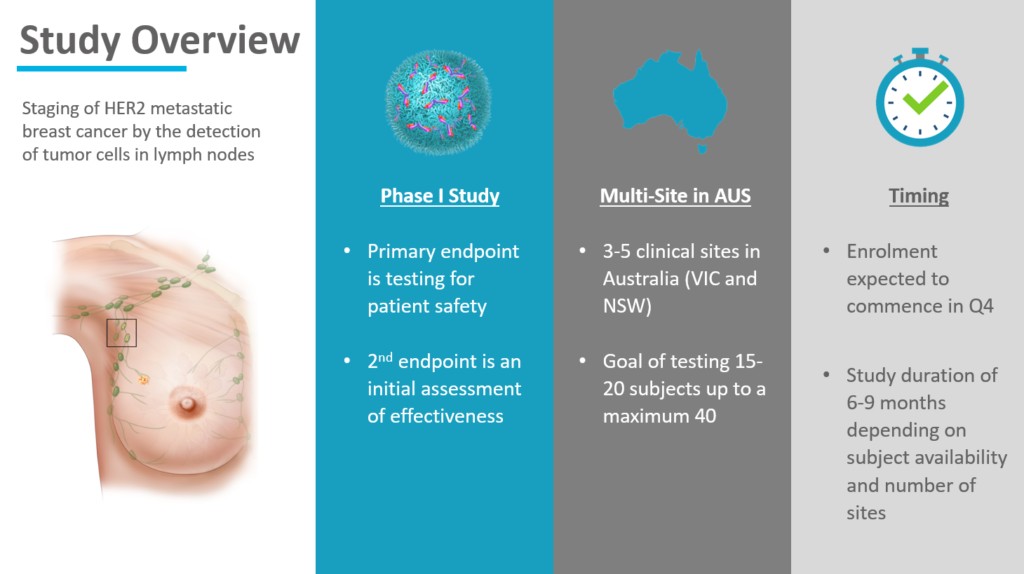
The Phase I study aims to evaluate the Company’s MagSense® HER2 nanoparticle test reagent for safety and initial effectiveness for lymph nodal metastases in HER2 breast cancer. The multi-site study is expected to enroll 15 to 20 patients, beginning in the fourth quarter of 2020, subject to Human Research Ethics Committee (HREC) approval and having study sites under contract.

“It is well known that there is a favorable regulatory and clinical testing environment for Phase I studies in Australia. For Imagion, there are a number of advantages to conducting the study in Australia, including a lower overall cost and the ability to expand the study to include MRI and still execute within our desired timeframes, thanks to the recently announced collaboration with Siemens Healthineers,” said Bob Proulx, Executive Chairman of Imagion Biosystems.

“While the primary objective of the study is to demonstrate that our nanoparticles are safe, it will also generate valuable data to show the effectiveness of our targeted nanoparticles in detecting the presence and spread of HER2 breast cancer using two different imaging modes. These study data will help us assess the best commercial pathway for improving nodal staging of HER2 breast cancer.
“We have worked hard to hold to our goal of entering the clinic this year. As previously reported, the first phase of manufacturing has been completed on time and the second phase continues on schedule, with no impact from the global pandemic so far. We are now focused on securing the clinical sites and obtaining the necessary clearances to commence the study.”
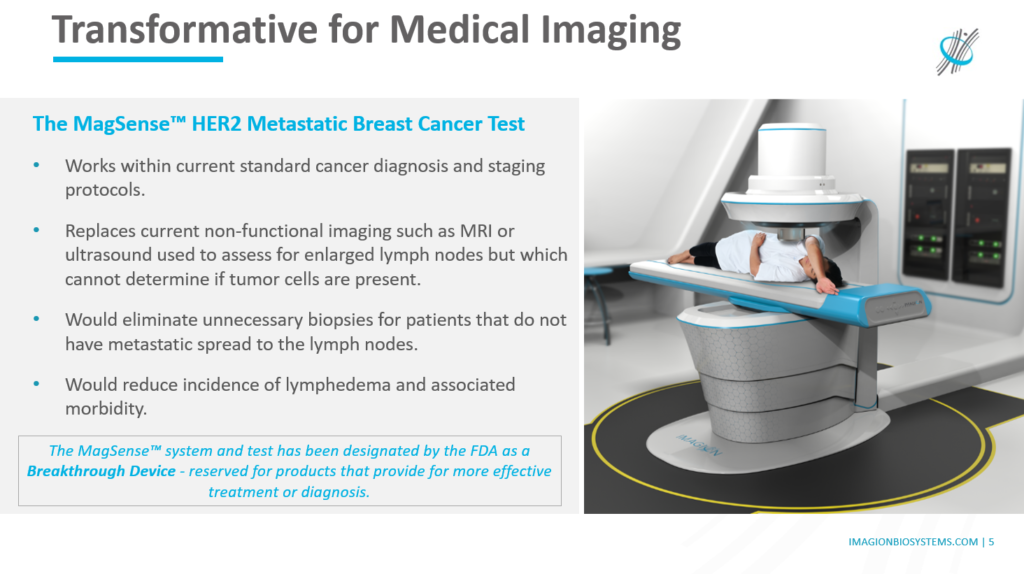
Imagion’s proprietary MagSense® technology works by using tiny bio-safe nanoparticles coated with tumour targeting antibodies to tag the cancer. MagSense® sensors are able to detect the particles which have become attached to cancer cells and which then act like a magnetic beacon. This imaging method, known as super magnetic relaxometry (SMPR), has been designated by the FDA as a Breakthrough Device for its potential to change cancer staging and diagnoses.
In the MRI setting, contrast agents are used to enhance the image and improve resolution but are typically general contrast agents and do not provide specific bio-functional information. Imagion’s MagSense® nanoparticles could provide an enhanced MR image of specific diseased tissue, thus improving on the ability to identify cancer tissues with existing imaging technology.
In May 2020, Imagion Biosystems entered into a collaboration agreement with Siemens Healthcare Pty Ltd of Australia (Siemens Healthineers) to further explore the utility of Imagion’s MagSense® nanoparticles as an MRI contrast agent.