Interested to learn more information about our independent review and path to market?
Highlights
MELBOURNE — Imagion Biosystems Limited (ASX: IBX), a company dedicated to improving healthcare through the earlier detection of cancer, is pleased to announce that an independent blinded review by a panel of expert breast cancer radiologists has corroborated the Company’s previously reported positive findings.
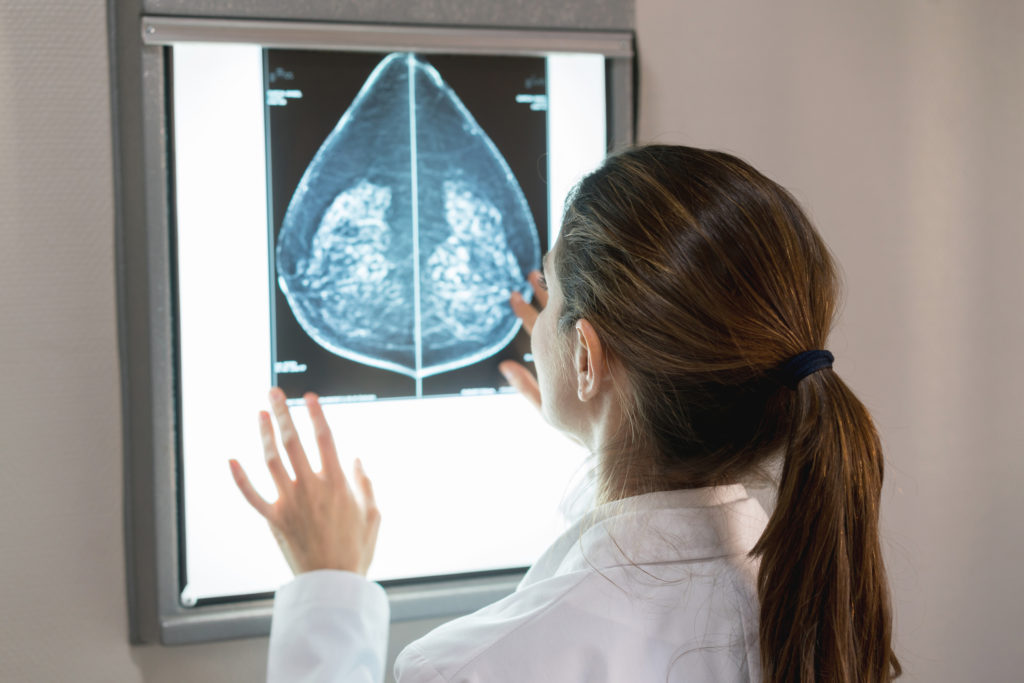
Each radiologist ascertained that the MagSense® HER2 imaging agent produces a change in image contrast and that the contrast in nodes highly suspicious for tumour is distinctly different from the MR image contrast seen in non-involved nodes. The independent reviews are consistent with the Company’s previous assertion that the MagSense® imaging agent provides new information for the radiologist not available through conventional methods, like ultrasound, and has the potential to aid in the clinical assessment of nodal metastasis in HER2 positive breast cancer.
The confirmation by the panel of the Company’s assessment that cancer detection can be achieved when using the MagSense® imaging agent with MRI has significant implications for the Company.
“The outcome of the independent review is welcome news, indeed,” stated Bob Proulx, CEO. “We now have a clear indication that our MagSense® magnetic nanoparticle technology could work with the existing medical imaging infrastructure to provide the clinical benefit to breast cancer patients we have been aiming for. This takes a lot of the technical risk out of the future and will significantly facilitate market entry by eliminating the need to design, make, sell, and support new machinery.”
Adding, “We will continue to refine our proprietary relaxometry technology but can now confidently shift our resources to developing our nanoparticles for use with MRI, a ubiquitous imaging modality used in hospitals and radiology clinics throughout the world. We believe this will be more attractive for strategic partners and more likely for us to achieve commercial success sooner.”
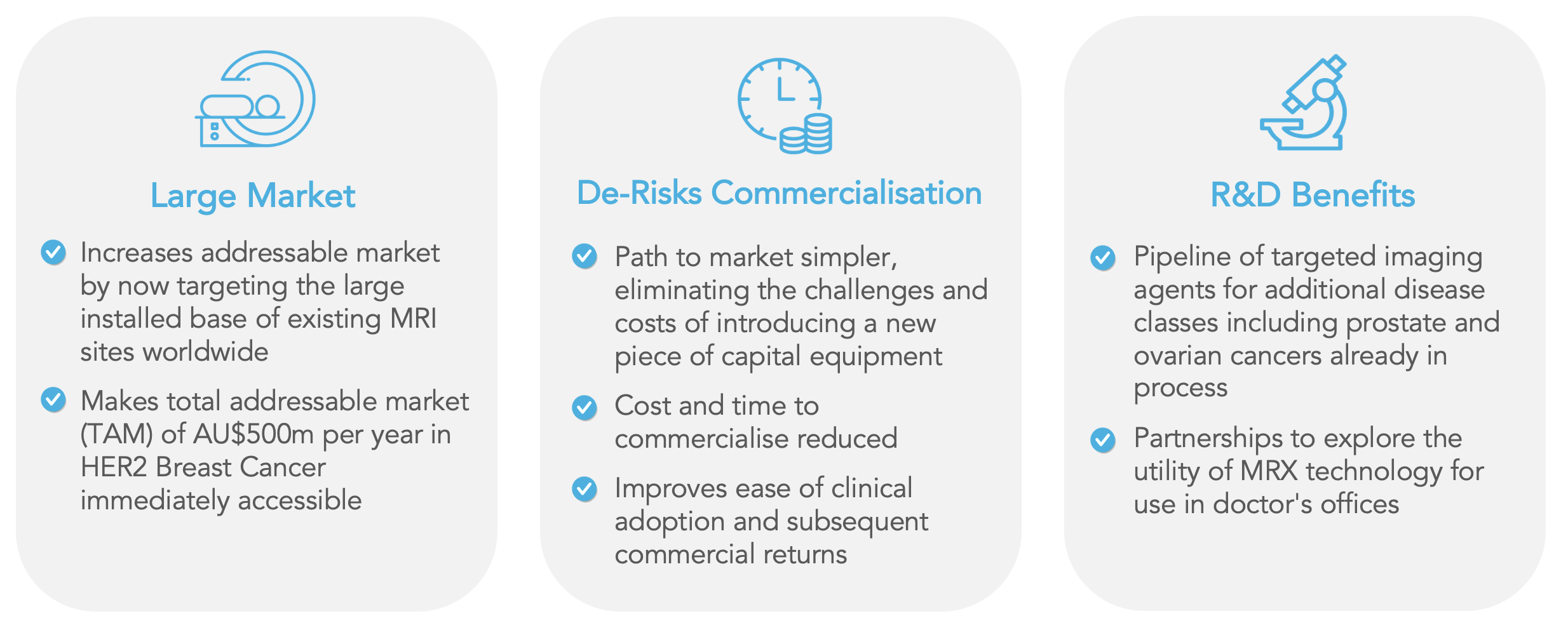
Since all MagSense® targeted imaging agents use the same underlying magnetic nanoparticle technology, the Company now plans to prioritize the development of its MagSense® nanoparticle technology for use with mainstream clinical MRI scanners.
The Company has already initiated the regulatory process with the US Food and Drug Administration (FDA) regarding bringing the HER2 Breast Cancer clinical studies to the US where there will be access to a larger number of sites and a more substantial patient population.
Whereas previously the FDA had designated the MagSense® relaxometry technology as a medical device, recent US legislation has stipulated that any MRI contrast agent be regulated as drugs through the imaging group within the Center for Drug Evaluation and Research (CDER). The Company does not expect the change to seek approval for its imaging agents for use with commercially available MRI scanners to significantly impact the time or cost associated with obtaining regulatory approvals.
We hosted a live Fireside Chat Webinar on Tuesday, February 7th to provide additional details and answer questions. You can catch the recording of it HERE.
About The Phase I Study and Data
Imagion’s MagSense® HER2 Breast Cancer Phase I study is the first clinical investigation of its proprietary MagSense® magnetic nanoparticle technology. The study was designed to be an initial proof-of-principle in human subjects to assess whether targeted magnetic nanoparticles can provide molecular imaging without the use of radiation in humans.
In this Phase I study, the MagSense® HER2 imaging agent is being investigated for its potential to detect HER2 positive breast cancer cells that may have metastasized to the lymph nodes. Each subject in the study receives an injection of the MagSense® HER2 targeted imaging agent and assessment by two imaging modalities: MRI and the Company’s proprietary magnetic relaxometry imaging (MRX) method.
In December Imagion reported a summary of the results for the first cohort of six patients, which included data demonstrating that the imaging agent was detectable by both imaging modalities and that MRI images from morphologically enlarged vs. normal nodes showed discriminating image features. Previously, the Company had announced that the study’s Safety Review Committee had completed a review of the first cohort with no safety or tolerability issues related to the imaging agent being reported. A total of nine (9) subjects have completed the study to-date with all safety and imaging results remaining consistent as reported for the first cohort. Combined, these findings indicate that the study is on track to meet its primary objectives.
For each patient participating in the study, the MR scans of the axillary region typically result in many nodes being imaged with varying amounts of tumour burden. Given the importance of the initial proof of principle for determining the future development of the product, the Company determined it would be prudent and timely to establish an independent expert panel of radiologists to review the MRI data. The panel undertook a blinded analysis of the pre- and post-dose MR images for six patients. Reviewers were tasked with confirming if there was a discernable difference in image contrast for nodes considered “suspicious” prior to the agent being administered compared to the nodes considered “normal” or absent of tumour cells. All reviewers noted a similar and particular pattern of image contrast that is identifiably different and may be attributed to cancer cells that metastasize to the lymph nodes.
“This independent review is very encouraging” said Dr Jane Fox, the study Principal Investigator. “The assessments by these experienced breast radiologists are in line with our thinking that these early patient results are showing that the MagSense® HER2 imaging agent is adding valuable information that can be used to better assess a patient’s medical condition before starting treatment or planning surgery.”
The Company plans to keep the study open for enrolment to continue to accumulate valuable information that may inform future study considerations and gather a larger data set that may be used for training purposes.
How MagSense® with MRI addresses the unmet need
After a new breast cancer diagnosis, the patient is assessed for metastatic disease, initially by nodal staging. This involves a combination of clinical assessment and radiographic imaging. Precise nodal staging is an essential component in the management of patients with breast cancer, as treatments depend on patient-specific characteristics of the primary tumor, nodal status, and evaluation for distant metastatic disease. The use of imaging varies widely based on available resources and institutional experience with ultrasound being the more common method for nodal assessment. However, ultrasound is limited to morphological assessment (node size and shape), is limited in the area that can be scanned, and is highly dependent on the experience of the operator. As a result, diagnostic sensitivity and specificity is highly variable and so whether there is a suspected presence of abnormal nodes or not, a patient will typically be required to have additional evaluation by biopsy and pathologic confirmation. Recently the breast cancer community has been making a push towards “de-escalation of axillary surgery”, i.e. reducing node removals and biopsies.
MRI is well suited to image soft tissues of the body, is less dependent on operator experience, and can scan the entire nodal region. However, it is also limited to identifying “regions of interest” based on anatomical or morphological abnormalities. Whilst current contrast agents, such as gadolinium, can improve general image signal-to-noise ratios they are non-specific, i.e. they do not target the molecular phenotype of cancer cells, and therefore cannot specify if tumour cells are present.
It is within this clinical context that Imagion’s noninvasive and molecularly targeted, tumor-specific approach can add value. With more than 30,000 clinical-grade scanners globally, MRI is a ubiquitous and accessible imaging technology. If brought to market for use in conjunction with such a well-accepted imaging modality, the Company’s MagSense® technology would be the first molecular imaging agent developed for Magnetic Resonance Imaging and can change the way we look at cancer.
The Company plans to continue to develop the proprietary detection technology, known as magnetic relaxometry (MRX) in collaboration with academic and industry partners, focusing on a new generation of small magnetic sensors that could enable the use of magnetic relaxometry in the doctor’s office.
In the future MRX could be a game-changing instrument that would allow non-invasive cancer diagnosis at the first point of care, e.g. at the urologist or OB/Gyn or general practitioner.
Key Points of the Strategy Shift
Based on the clinical data and the independent review, the Company believes the most appropriate commercial strategy is to prioritize the development of its MagSense® nanoparticle technology as imaging agents to be used with mainstream clinical MRI scanners.
The Company would like to acknowledge and thank the patients that have enrolled in the Phase I study to date, without whom we would not be able to advance our idea of improving cancer detection. Additionally, we would like to acknowledge the Company’s founder, Dr. Edward Flynn, for his foresight and for pioneering the idea that magnetic nanoparticles could be used in a targeted way to non-invasively detect cancers.
Imagion Biosystems is developing a new non-radioactive and safe diagnostic imaging technology. Combining biotechnology and nanotechnology, the Company aims to detect cancer and other diseases earlier and with higher specificity than is currently possible. Imagion Biosystems listed on the Australian Securities Exchange (ASX) in June 2017.

PDF of the ASX announcement HERE.
Questions about this announcement or other investor-related inquiries? Please email our investor relations department at: investor@imagionbio.com.
Stay informed about our latest news and developments.
© Imagion Biosystems, Ltd. All Rights Reserved | Privacy Policy | Terms & Conditions | Careers | Corporate Governance
